Reactive oxygen species (ROS) in excess have been documented in active vitiligo skin. Numerous proteins in addition to tyrosinase are affected. It is possible that oxidative stress is one among the main principal causes of vitiligo. However, there also exists ample evidence for altered immunological processes in vitiligo, particularly in chronic and progressive conditions. Both innate and adaptive arms of the immune system appear to be involved as a primary event or as a secondary promotive consequence.The article focuses on the linking oxidative stress and immune system to vitiligo pathogenesis giving credence to a convergent terminal pathway of oxidative stress–autoimmunity-mediated melanocyte loss.
Oxidative stress is essentially an imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects through neutralization by antioxidants.
Oxidative stress leads to many pathophysiological conditions in the body. Some of these include neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease, gene mutations and cancers, chronic fatigue syndrome, fragile X syndrome, heart and blood vessel disorders, atherosclerosis, heart failure, heart attack and inflammatory diseases.
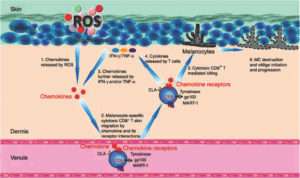
Vitiligo is a common skin disorder characterized by the acquired loss of constitutional pigmentation manifesting as white macules and patches caused by a loss of functioning epidermal melanocytes.There is speculation on the interplay, if any, between ROS(reactive oxygen species) and the immune system in the pathogenesis of vitiligo.
Schallreuter KU et al, (1) said in patients with vitiligo the epidermis contains increased levels of ROS, mainly H2O2 and peroxynitrite, as well as inadequate antioxidant defences. It affects 0.5–1% of the world population, and its incidence ranges from 0.1 to 8.8% in India.
A single dominant pathway appears unlikely to account for all cases of melanocyte loss in vitiligo, and apparently, a complex interaction between genetic, environmental, biochemical and immunological events is likely to generate a permissive milieu
Human skin is constantly exposed to a broad array of physical, chemical and biological agents, many of which are either inherent oxidants or catalyse the generation of ROS. A lot of studies suggested that an hypersensitivity to oxidative stress has a crucial role in determining melanocyte degeneration [2].
Hann SK et al said that there is an elevated activity of NADPH oxidase and NOS (Nitric oxide synthase), with secondary increase production of ROS and reactive nitrogen species [3]
High levels of the tetrahydrobiopterin (6BH4) and its isomer 7BH4, has been demonstrated in vitiligo epidermis [4,5]. Tetra-hydrobiopterin, an essential cofactor for the aromatic amino acid hydroxylases and NOS. Increased biopterin levels boost the formation of H2O2 and inhibit the function of phenylalanine and tyrosine hydroxylases, thus impairing melanin production in melanocytes and inducing norepinephrine accumulation in keratinocytes [6].Biopterins act as inhibitors of the enzymes involved in melanogenesis (namely, phenylalanine hydroxylase and tyrosinase) and stimulate the formation of H2O2.
A systemic redox defect in this disease which is characterised bylow levels of enzyme catalase,an antioxidant enzyme that catalyzes the conversion of hydrogen peroxide in water plus oxygen, and other antioxidant agents such as glutathione peroxidase, glucose-6-phosphate dehydrogenase, superoxide dismutase, and vitamins C and E have been detected both in the epidermis or in the serum of vitiligo patients.
A systemic redox defect might also be the consequence of an impaired mitochondrial functioning have been demonstrated in the epidermis of vitiligo skin biopsies. Such structural defects directly correlate with a consequent impaired mitochondrial activity, thus leading to an increased generation of reactive oxygen species.
Oxidative stress is reported to play a role in progress of vitiligo but there is conflicting evidence for the same. Some researchers report increased total antioxidant levels,others report no change or even decreased levels of these markers like Superoxide Dismutase(SOD), Glutathione peroxidase (GPx), Malondialdehyde (MDA),Nitric Oxide (NO), and Catalase.(7)
Current literature reports several evidences suggesting a strict interplay between oxidative stress and immune system, able to trigger and maintain vitiligo depigmentation process and the eventually associated autoimmune thyroid disorders ATD [8].
The role played by autoimmunity and oxidative stress in the pathogenesis of vitiligo until now was considered as mutually exclusive. Recent findings instead suggested that these two mechanisms are both involved in the depigmentation process and act in synergism [8]. In autoimmune disorders such as vitiligo, the immune system develops a chronic inflammatory milieu in which ROS accumulate and exert a toxic effect on surrounding cells [8].
Structural or functional melanocytic proteins therefore may be modified by acute and chronic oxidative stress, possibly becoming neoantigens able to trigger autoreactive reactions [9]. Hence, according to this new theory, autoimmunity and oxidative stress interact in initiating and/or amplifying the loss of melanocytes in vitiligo.
Overall, according to the evidences and theories discussed above, we can state that vitiligo has complex pathogenesis in which a pivotal role is played by oxidative stress and immune system. A growing body of evidences indeed shows that autoimmunity and oxidative stress interact and work together in creating a pathway finally able to determine melanocyte loss.
References :
- Schallreuter KU, Moore J, Wood JM, Beazley WD, Gaze DC, Tobin DJ, Marshall HS, Panske A, Panzig E, Hibberts NA (1999) In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase.JInvestig DermatolSympProcSoc Investig DermatolInc EurSoc Dermatol Res 4(1): 91 –96
- V. Maresca, M. Roccella, F. Roccella et al., “Increased sensitivity to peroxidative agents as a possible pathogenic factor of melanocyte damage in vitiligo,” Journal of Investigative Dermatology, vol. 109, no. 3, pp. 310–313, 1997
- Hann SK, Chang JH, Lee HS, Kim SM (2000) The classification of segmental vitiligo on the face. Yonsei Med J 41(2):209–212. doi:10.3349/ymj.2000.41.2.209
- K. U. Schallreuter, J. M. Wood, I. Ziegler et al., “Defective tetrahydrobiopterin and catecholamine biosynthesis in the depigmentation disorder vitiligo,” Biochimica et BiophysicaActa, vol. 1226, no. 2, pp. 181–192, 1994. no. 4, pp. 549–556, 2006.
- S. Hasse, N. C. J. Gibbons, H. Rokos, L. K. Marles, and K. U. Schallreuter, “Perturbed 6-tetrahydrobiopterin recycling via decreased dihydropteridinereductase in vitiligo: more evidence for H2O2 stress,”
- Schallreuter KU, Wood JM, Ziegler I, Lemke KR, Pittelkow MR, Lindsey NJ, Gutlich M (1994) Defective tetrahydrobiopterin and catecholamine biosynthesis in the depigmentation disorder vitiligo. BiochimBiophysActa 1226(2):181–191
- Anju Jain Jyoti Mal VibhuMehndiratta Ram Chander Surajeet Kumar Patra,”Study of Oxidative Stress in Vitiligo”Ind J ClinBiochem (Jan-Mar 2011) 26(1):78–81
- 13.N. C. Laddha, M. Dwivedi, M. S. Mansuri et al., “Vitiligo: interplay between oxidative stress and immune system,” Experimental Dermatology, vol. 22, no. 4, pp. 245–250, 2013.
- B. T. Kurien, K. Hensley, M. Bachmann, and R. H. Scofield, “Oxidatively modified autoantigens in autoimmune diseases,” Free Radical Biology and Medicine, vol. 41, no. 4, pp. 549–556, 2006